- TOLL-FREE HELPLINE: 1-800-659-1991
- CONTACT US
- Supporting Families Affected by Prion Disease
The status of prion disease drug development changes regularly. In July 2025, five organizations working toward a treatment for prion disease met with families and spoke on a panel at the CJD Foundation Family Conference in Chicago. Hear more about the information each of those organizations shared at the conference.
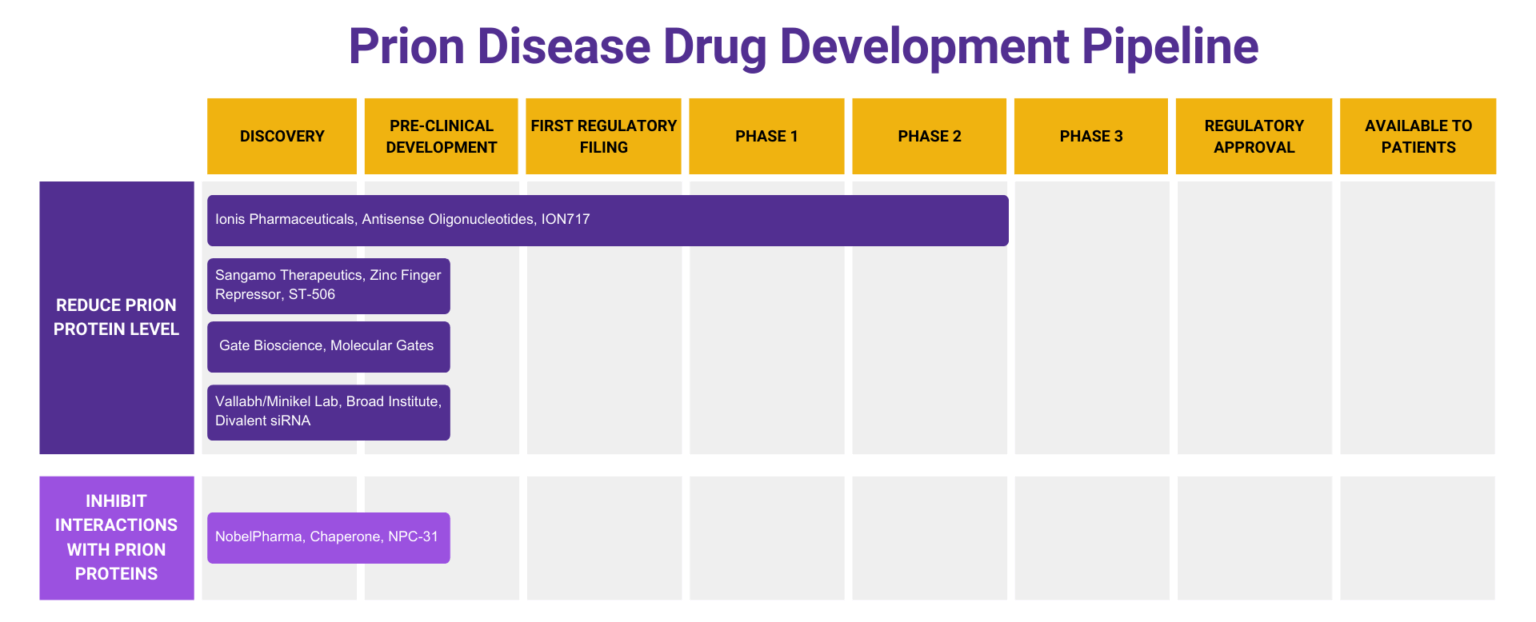
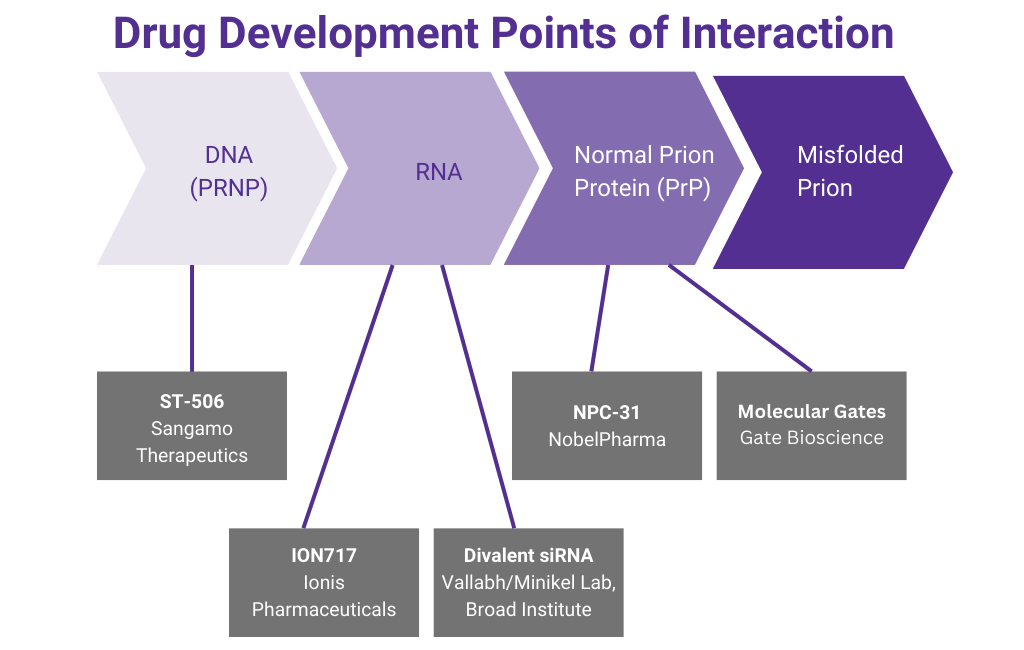




Learn about antisense oligonucleotide targeting of PRNP mRNA as a potential treatment for prion disease from this recording of the 2020 Virtual Conference Session presented by Anne Smith, PhD, Executive Director of Clinical Development at Ionis Pharmaceuticals, a California-based biotechnology company focused on RNA-targeting therapeutics.
Launched in Fall 2015, Prions@Broad represents an innovative new model for rare disease drug discovery. Sonia Vallabh and Eric Minikel changed careers to become scientists and devote their lives to prion therapeutics after learning, in December 2011, that Sonia had inherited a fatal genetic mutation in the prion protein gene from her late mother. In our unique model for precision medicine research, these two personally motivated patient-scientists with their disease-specific focus are embedded within a department and an institute equipped with the tools and expertise needed to enable therapeutic discovery.
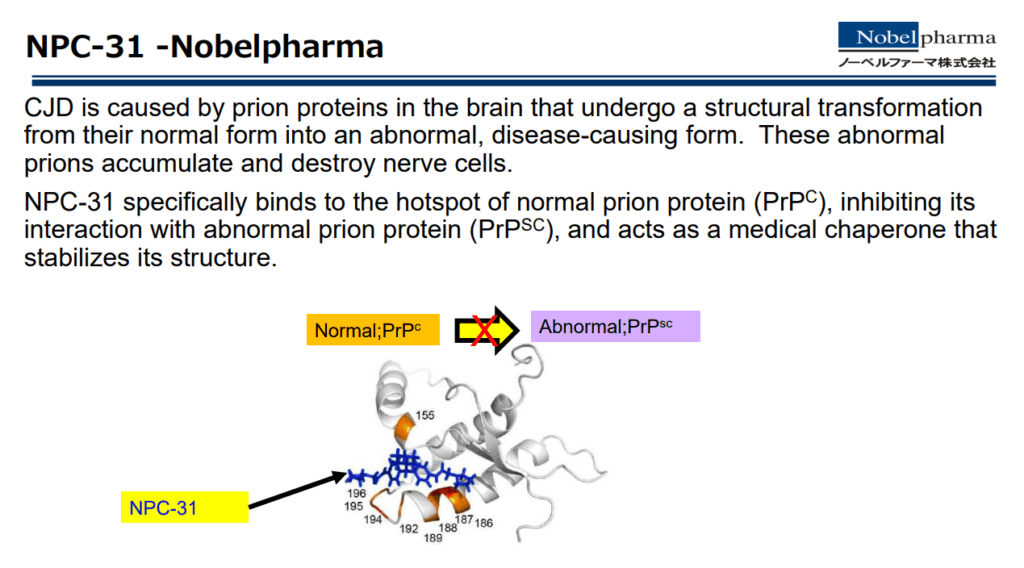
Nobelpharma has constantly been aspiring to conduct research and development of drugs, that are not the prime targets for many pharmaceutical companies due to questionable economic viability with a small number of patients (drugs for unmet medical needs).
“We have completed several essential non-clinical studies and expect to initiate clinical studies very soon, probably next year.”
— Kiyoshi Arita, MSc, Project/Product Manager, Research and Development Division, International Clinical Development Department, Nobelpharma Co., Ltd., Tôkyô, Japan, speaking at CJD Foundation Family conference, July 2025
Learn about a potential future treatment for prion disease from this recording of the 2022 presentation by Brian Zeitler, Senior Director of Gene Regulation, Sangamo Therapeutics: “Engineered zinc finger protein transcription factors potently reduce brain PrP expression and extend survival in prion-infected mice”
Learn about the process of clinical research and drug development and the role rare disease patient communities can play in the process from this recording of the 2022 Virtual Conference Session presented by Laura Iliescu, MSc, Director, Patient Advocacy Strategy, Center for Rare Diseases.